Homemade Hand Sanitizer Is Easy to Make. However Do Not Make It. Here's Why...
One of the most popular recipes out there is:
- 150 ml alcohol
- 75 ml aloe vera
- 10ml tea tree oil
Vodka is frequently suggested as a good alcohol to use. The flaw with this is that hand sanitizer has to have an alcohol concentration of 60% or more to be effective and meet infecion control standards. Vodka is typically 40%, so with this recipe, if you have 150 ml of vodka, the amount of alcohol in this is 60ml. Once you add the vodka to the aloe vera gel, you have a total of 225ml. If you have 60ml of alcohol, the percentage of alcohol in this mixture is only 26.66%, which isn’t even half of the recommended concentration. You would have to have an alcohol with a much higher concentration than vodka to get a high enough percentage. Even using another alcohol like 99% isopropyl alcohol doesn’t work. Your alcohol concentration would work out to 65.7%, but you still don’t know if it would work as a sanitizer, plus it would be very drying on your skin.
Another factor in this, is that aloe vera gel loses it’s thick, gel like qualities in this concentration of alcohol and breaks down affecting the consistency of your gel.
Tea tree is a fantastic essential oil with many qualities, it is believed to have antiviral, anti-inflammatory, antiviral and antifungal properties, but that does not mean that it is effective against all viruses.
Companies that produce hand sanitizers have to go through rigid standards and testing to consistently produce products that are safe and that deliver the results that are claimed by the company. There is a reason that they won’t share their formulas with the public and that is that they have a duty to keep us safe.
Hand sanitizers have their uses, but still the most effective hand hygiene method is hand washing. The soap actually destroys the virus.

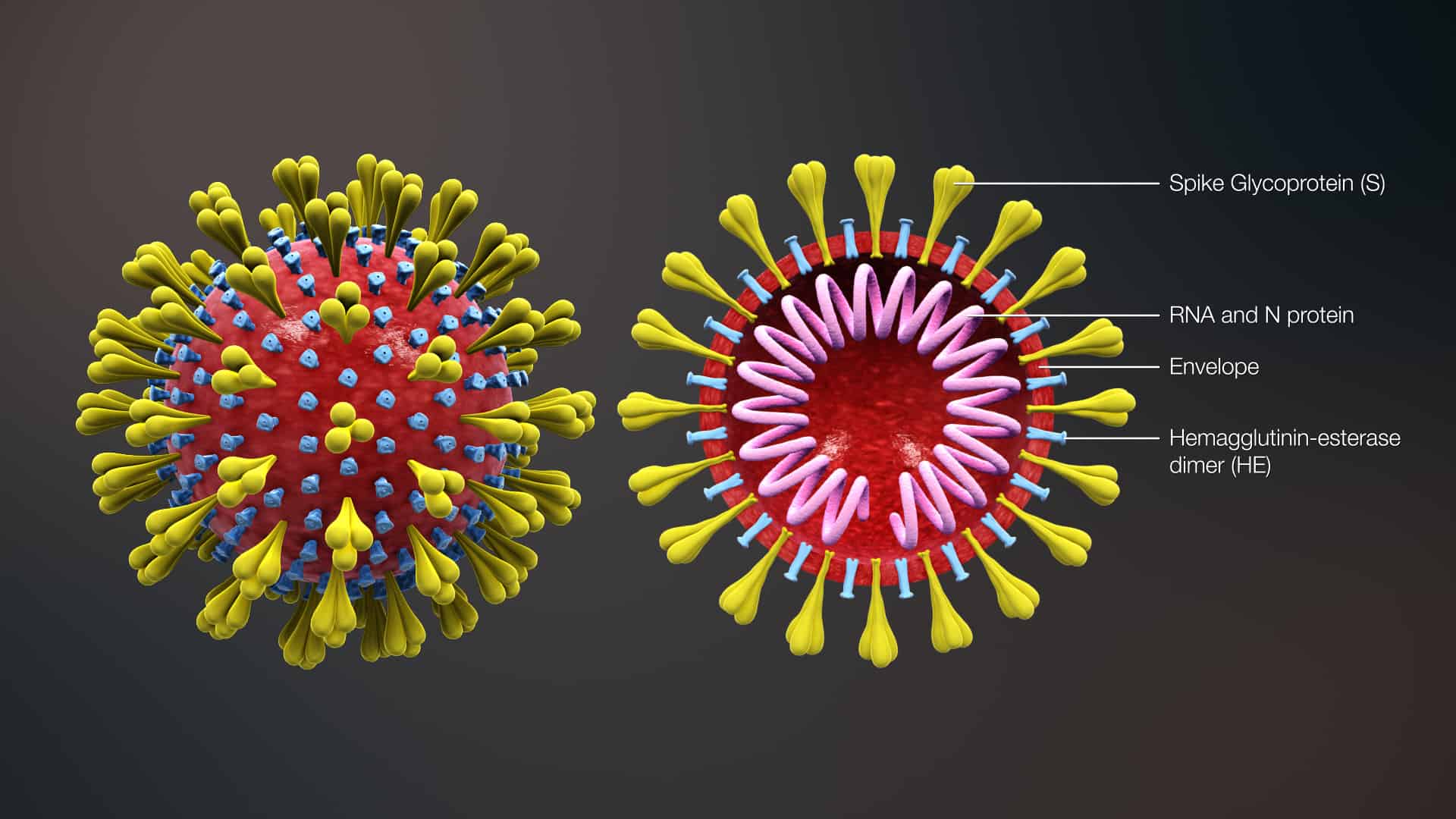
The virus has a fatty outer layer
Soap breaks down this outer layer. Think of the virus as a drop of oil and imagine if you had a beaker of water and poured some oil on the top. The oil would just stay there, floating on the top. If, however, you added soap to it the fat would mix with the water.

Soap breaks down this outer layer. Think of the virus as a drop of oil and imagine if you had a beaker of water and poured some oil on the top. The oil would just stay there, floating on the top. If, however, you added soap to it the fat would mix with the water.
Soap molecules are bipolar, meaning the molecules have oppositely charged ends. One end of the molecule is negatively-charged and hydrophobic. It is repelled by water because water is also negatively charged – think of trying to push the same poles of a magnet together.
However, this hydrophobic end is attracted to fats and it grabs on to them from every side. The soap literally pulls the fatty layer apart into the water and it washes away. This takes time though – rinsing with plain water, or only washing for 5 or 10 seconds is not enough to break down the fat. You need to wash for at least 20 seconds in order for the soap to grab on to all the fats.
The other end of the soap molecule is positively-charged and hydrophilic. It is attracted to water. During rinsing, the water carries away the fats. This is why rinsing is a crucial step to the cleaning process.
If you want to add tea tree oil to add extra qualities to your soap, go ahead. Any fragrance is fine though – it is the basic qualities of the soap that destroy the virus – even a plain unfragranced soap is effective.

If you want to add tea tree oil to add extra qualities to your soap, go ahead. Any fragrance is fine though – it is the basic qualities of the soap that destroy the virus –even a plain unfragranced soap is effective.
Hand sanitizer is effective, especially when you are out and about and have no access to soap and water. However, the CDC is recommending washing with soap and water whenever you can, especially if you have visibly soiled hands.
As soap makers this is our time to shine! We have the perfect weapon against the coronavirus, and the perfect excuse to make lots of yummy soap!

0 comments